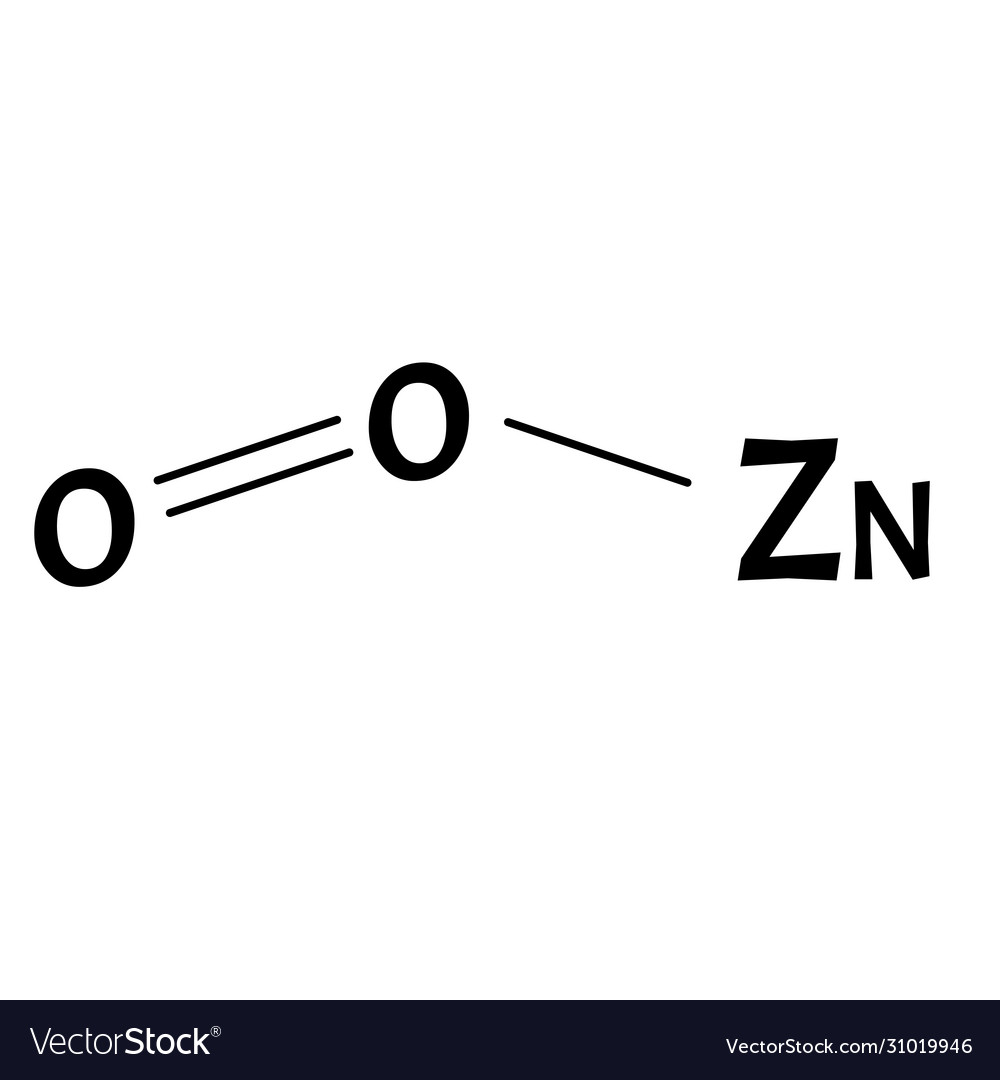
Zinc Copper(Ii) Oxide Equation . C + 2cuo co 2 + 2cu. Web copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Web in the first reaction, the copper ion is able to oxidize the zinc metal. Cu + zno = cuo + zn is a single displacement (substitution) reaction. If something gains oxygen, it is said to have been oxidised. Web aluminium is oxidised because it gains oxygen to form aluminium oxide. Web this is the easy bit! Zinc oxide acts as an oxidising agent because it can. For each of the following experiments. Web zinc + copper(ii) oxide → zinc oxide + copper zn(s) + cuo(s) → zno(s) + cu(s) questions. Web copper + zinc oxide = copper (ii) oxide + zinc. However, in the second reaction, the zinc ion is not able to. By observing this reaction and its products,. Web the reaction between zinc and 42 copper oxide in this experiment copper(ii) oxide and zinc metal are reacted together.
from www.vectorstock.com
However, in the second reaction, the zinc ion is not able to. Web in the first reaction, the copper ion is able to oxidize the zinc metal. C + 2cuo co 2 + 2cu. Web this is the easy bit! Cu + zno = cuo + zn is a single displacement (substitution) reaction. If something gains oxygen, it is said to have been oxidised. Web copper + zinc oxide = copper (ii) oxide + zinc. Web copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Web zinc + copper(ii) oxide → zinc oxide + copper zn(s) + cuo(s) → zno(s) + cu(s) questions. By observing this reaction and its products,.
Zinc oxide is a molecular chemical formula Vector Image
Zinc Copper(Ii) Oxide Equation C + 2cuo co 2 + 2cu. Web in the first reaction, the copper ion is able to oxidize the zinc metal. By observing this reaction and its products,. Cu + zno = cuo + zn is a single displacement (substitution) reaction. If something gains oxygen, it is said to have been oxidised. Web zinc + copper(ii) oxide → zinc oxide + copper zn(s) + cuo(s) → zno(s) + cu(s) questions. For each of the following experiments. Web copper + zinc oxide = copper (ii) oxide + zinc. However, in the second reaction, the zinc ion is not able to. Web the reaction between zinc and 42 copper oxide in this experiment copper(ii) oxide and zinc metal are reacted together. C + 2cuo co 2 + 2cu. Web this is the easy bit! Web aluminium is oxidised because it gains oxygen to form aluminium oxide. Web copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Zinc oxide acts as an oxidising agent because it can.
From www.fity.club
Copper Ii Zinc Copper(Ii) Oxide Equation However, in the second reaction, the zinc ion is not able to. For each of the following experiments. Zinc oxide acts as an oxidising agent because it can. Web the reaction between zinc and 42 copper oxide in this experiment copper(ii) oxide and zinc metal are reacted together. Web copper (ii) oxide and zinc metal react together in an exothermic. Zinc Copper(Ii) Oxide Equation.
From edu.rsc.org
The reaction between zinc and copper(II) oxide Resource RSC Education Zinc Copper(Ii) Oxide Equation Web in the first reaction, the copper ion is able to oxidize the zinc metal. Web copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. For each of the following experiments. C + 2cuo co 2 + 2cu. However, in the second reaction, the zinc ion is not able to. Web. Zinc Copper(Ii) Oxide Equation.
From www.slideserve.com
PPT Writing Formulas and Names of Ionic Compounds PowerPoint Zinc Copper(Ii) Oxide Equation Zinc oxide acts as an oxidising agent because it can. Web copper + zinc oxide = copper (ii) oxide + zinc. Cu + zno = cuo + zn is a single displacement (substitution) reaction. C + 2cuo co 2 + 2cu. By observing this reaction and its products,. For each of the following experiments. However, in the second reaction, the. Zinc Copper(Ii) Oxide Equation.
From armsingle10.pythonanywhere.com
Peerless Molecular Formula Of Rust Write The Balanced Equation Zinc Copper(Ii) Oxide Equation Web copper + zinc oxide = copper (ii) oxide + zinc. C + 2cuo co 2 + 2cu. For each of the following experiments. By observing this reaction and its products,. Zinc oxide acts as an oxidising agent because it can. If something gains oxygen, it is said to have been oxidised. Web zinc + copper(ii) oxide → zinc oxide. Zinc Copper(Ii) Oxide Equation.
From www.biosynth.com
FC160925 1317380 Copper(II) oxide Biosynth Zinc Copper(Ii) Oxide Equation C + 2cuo co 2 + 2cu. Web in the first reaction, the copper ion is able to oxidize the zinc metal. Web aluminium is oxidised because it gains oxygen to form aluminium oxide. Cu + zno = cuo + zn is a single displacement (substitution) reaction. Web zinc + copper(ii) oxide → zinc oxide + copper zn(s) + cuo(s). Zinc Copper(Ii) Oxide Equation.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc Copper(Ii) Oxide Equation If something gains oxygen, it is said to have been oxidised. Cu + zno = cuo + zn is a single displacement (substitution) reaction. C + 2cuo co 2 + 2cu. By observing this reaction and its products,. However, in the second reaction, the zinc ion is not able to. Web copper + zinc oxide = copper (ii) oxide +. Zinc Copper(Ii) Oxide Equation.
From brainly.in
Write chemical equation for obtaining zinc metal from zinc sulphide Zinc Copper(Ii) Oxide Equation Web this is the easy bit! Web zinc + copper(ii) oxide → zinc oxide + copper zn(s) + cuo(s) → zno(s) + cu(s) questions. Web copper + zinc oxide = copper (ii) oxide + zinc. For each of the following experiments. Cu + zno = cuo + zn is a single displacement (substitution) reaction. By observing this reaction and its. Zinc Copper(Ii) Oxide Equation.
From www.slideshare.net
Chapter 4 Zinc Copper(Ii) Oxide Equation Cu + zno = cuo + zn is a single displacement (substitution) reaction. For each of the following experiments. Web zinc + copper(ii) oxide → zinc oxide + copper zn(s) + cuo(s) → zno(s) + cu(s) questions. Web copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. If something gains oxygen,. Zinc Copper(Ii) Oxide Equation.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) Zinc Copper(Ii) Oxide Equation However, in the second reaction, the zinc ion is not able to. Web copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. If something gains oxygen, it is said to have been oxidised. C + 2cuo co 2 + 2cu. For each of the following experiments. Web in the first reaction,. Zinc Copper(Ii) Oxide Equation.
From www.studocu.com
Empirical formula of copper oxide 1 Empirical Formula of Copper Oxide Zinc Copper(Ii) Oxide Equation Web copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. By observing this reaction and its products,. Web zinc + copper(ii) oxide → zinc oxide + copper zn(s) + cuo(s) → zno(s) + cu(s) questions. Cu + zno = cuo + zn is a single displacement (substitution) reaction. Web the reaction. Zinc Copper(Ii) Oxide Equation.
From www.fishersci.com
Copper(II) oxide, Puratronic , 99.995 (metals basis), Thermo Zinc Copper(Ii) Oxide Equation If something gains oxygen, it is said to have been oxidised. By observing this reaction and its products,. Web the reaction between zinc and 42 copper oxide in this experiment copper(ii) oxide and zinc metal are reacted together. C + 2cuo co 2 + 2cu. Zinc oxide acts as an oxidising agent because it can. Web zinc + copper(ii) oxide. Zinc Copper(Ii) Oxide Equation.
From encyclopedia.pub
Zinc Oxide Doped with Transition Metal Ions Encyclopedia MDPI Zinc Copper(Ii) Oxide Equation For each of the following experiments. Web this is the easy bit! However, in the second reaction, the zinc ion is not able to. C + 2cuo co 2 + 2cu. If something gains oxygen, it is said to have been oxidised. By observing this reaction and its products,. Web in the first reaction, the copper ion is able to. Zinc Copper(Ii) Oxide Equation.
From www.vectorstock.com
Zinc oxide is a molecular chemical formula Vector Image Zinc Copper(Ii) Oxide Equation Web aluminium is oxidised because it gains oxygen to form aluminium oxide. Web copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Web zinc + copper(ii) oxide → zinc oxide + copper zn(s) + cuo(s) → zno(s) + cu(s) questions. By observing this reaction and its products,. Web this is the. Zinc Copper(Ii) Oxide Equation.
From www.toppr.com
Write balanced chemical equations for the following word equation Zinc Copper(Ii) Oxide Equation Zinc oxide acts as an oxidising agent because it can. Web in the first reaction, the copper ion is able to oxidize the zinc metal. Cu + zno = cuo + zn is a single displacement (substitution) reaction. Web this is the easy bit! Web copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc. Zinc Copper(Ii) Oxide Equation.
From www.vrogue.co
Hydrogen Gas Balanced Equation For Hydrogen Gas vrogue.co Zinc Copper(Ii) Oxide Equation Web in the first reaction, the copper ion is able to oxidize the zinc metal. Cu + zno = cuo + zn is a single displacement (substitution) reaction. Web aluminium is oxidised because it gains oxygen to form aluminium oxide. Web copper + zinc oxide = copper (ii) oxide + zinc. However, in the second reaction, the zinc ion is. Zinc Copper(Ii) Oxide Equation.
From studylib.net
Empirical formula of copper oxidestudent Zinc Copper(Ii) Oxide Equation Web this is the easy bit! However, in the second reaction, the zinc ion is not able to. For each of the following experiments. By observing this reaction and its products,. Cu + zno = cuo + zn is a single displacement (substitution) reaction. Web aluminium is oxidised because it gains oxygen to form aluminium oxide. Web copper (ii) oxide. Zinc Copper(Ii) Oxide Equation.
From www.dreamstime.com
Zinc Oxide Chemical Formula. Vector Illustration. Stock Image Stock Zinc Copper(Ii) Oxide Equation Web this is the easy bit! By observing this reaction and its products,. Web copper + zinc oxide = copper (ii) oxide + zinc. Web zinc + copper(ii) oxide → zinc oxide + copper zn(s) + cuo(s) → zno(s) + cu(s) questions. If something gains oxygen, it is said to have been oxidised. Web copper (ii) oxide and zinc metal. Zinc Copper(Ii) Oxide Equation.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Zinc Copper(Ii) Oxide Equation Web aluminium is oxidised because it gains oxygen to form aluminium oxide. Web copper (ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Web this is the easy bit! For each of the following experiments. Zinc oxide acts as an oxidising agent because it can. Web in the first reaction, the copper. Zinc Copper(Ii) Oxide Equation.